Question: Consider the extraction of Mn+ from aqueous solution into organic solution by reaction with protonated ligand, HL: Rewrite Equation 22-13 in terms of Kextraction and
Consider the extraction of Mn+ from aqueous solution into organic solution by reaction with protonated ligand, HL:
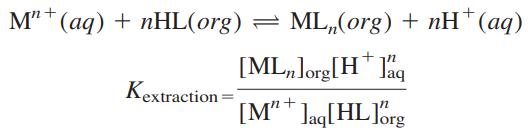
Rewrite Equation 22-13 in terms of Kextraction and express Kextraction in terms of the constants in Equation 22-13. Give a physical reason why each constant increases or decreases Kextraction.
Equation 22-13
![M"*(aq) + nHL(org) = ML(org) + nH*(aq) [MLJorg[H* Tag [M"+ laq[HL]org Kextraction=](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1592/8/1/2/1475ef06273bb8311592812130585.jpg)
M"*(aq) + nHL(org) = ML(org) + nH*(aq) [MLJorg[H* Tag [M"+ laq[HL]org Kextraction=
Step by Step Solution
3.26 Rating (167 Votes )
There are 3 Steps involved in it
Comparing the result to Equation 2213 gives Kextraction Constant Effect on Extraction Reason K ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
878-E-C-E-E-C (2268).docx
120 KBs Word File


