Question: Penicillin is produced by fermentation and recovered from the resulting aqueous broth by extraction with butyl acetate. The penicillin distribution coefficient K (mass fraction of
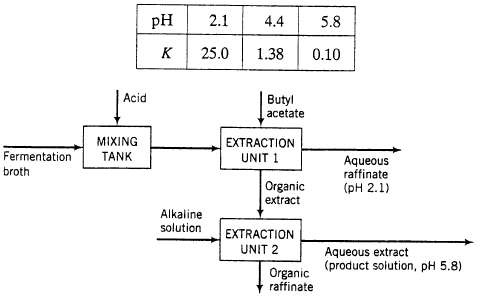
Penicillin is produced by fermentation and recovered from the resulting aqueous broth by extraction with butyl acetate. The penicillin distribution coefficient K (mass fraction of penicillin in the butyl acetate phase/mass fraction of penicillin in the water phase) depends strongly on the pH in the aqueous phase: Penicillin is produced by fermentation and recovered from the resulting aqueous broth by extraction with butyl acetate. The penicillin distribution coefficient K (mass fraction of penicillin in the butyl acetate phase/mass fraction of penicillin in the water phase) depends strongly on the pH in the aqueous phase:
This dependence provides the basis for the process to be described. Water and butyl acetate may be considered immiscible. The extraction is performed in the following three-unit process:
- Broth from a ferment or containing dissolved penicillin, other dissolved impurities, and water is acidified in a mixing tank. The acidified broth, which contains 1.5 wt% penicillin is contacted with liquid butyl acetate in an extraction unit consisting of a mixer, in which the aqueous and organic phases are brought into intimate contact with each other, followed by a settling tank, in which the two phases separate under the influence of gravity. The pH of the aqueous phase in the extraction unit equals 2.1. In the mixer 90% of the penicillin in the feed broth transfers from the aqueous phase to the organic phase
- The two streams leaving the settler are in equilibrium with each other?that is, the ratio of the penicillin mass fractions in the two phases equals the value of K corresponding to the pH of the aqueous phase (= 2.1 in Unit 1). The impurities in the feed broth remain in the aqueous phase. The raffinate (by definition, the product stream containing the feed-solution solvent) leaving Extraction Unit 1 is sent elsewhere for further processing, and the organic extract (the product stream containing the extracting solvent) is sent to a second mixer?settler Unit.
- In the second unit, the organic solution fed to the mixing stage is contacted with an alkaline aqueous solution that adjusts the pH of the aqueous phase in the unit to 5.8. In the mixer, 90% of the penicillin entering in the organic feed solution transfers o the aqueous phase. Once again, the two streams emerging from the settler are in equilibrium. The aqueous extract is the process product.
(a) Taking a basis of 100 kg of acidified broth fed to the first extraction Unit, draw and completely label a flowchart of this process and carry out the degree-of-freedom analysis to show that all labeled variables can be determined. (Suggestion: Consider the combination of water, impurities, and acid as a single species and the alkaline solution as a second single species, since the components of these ?pseudo species? always stay together in the process.)
(b) Calculate the ratios (kg butyl acetate required/kg acidified broth) and (kg alkaline solution required/kg acidified broth) and the mass fraction of penicillin in the product solution.
(c) Briefly explain the following:
(i) What is the likely reason for transferring most of the penicillin from an aqueous phase to an organic phase and then transferring most of it back to an aqueous phase, when each transfer leads to a loss of some of the drug?
(ii) What is the purpose of acidifying the broth prior to the first extraction stage, and why is the extracting solution added to the second unit a base?
(iii) Why are the two ?raffinates? in the process the aqueous phase leaving the first unit and the organic phase leaving the second unit, and vice versa for the ?extracts?? (Look again at the definitions of these terms.)
Acid MIXING Fermentation . broth pH 2.1 K 25.0 Alkaline solution 4.4 1.38 Butyl acetate EXTRACTION UNIT 1 Organic extract EXTRACTION UNIT 2 5.8 0.10 Organic raffinate Aqueous raffinate (pH 2.1) Aqueous extract (product solution, pH 5.8)
Step by Step Solution
3.35 Rating (164 Votes )
There are 3 Steps involved in it
a Ppenicillin Acacid solution BAbutyl acetate Alkalkaline solution Broth Acid DF analysis Extraction ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (344).docx
120 KBs Word File


