Question: Derive (15-44). Use this equation to solve the following problem. Sulfate ion is to be removed from 60 L of water by exchanging it with
Derive (15-44). Use this equation to solve the following problem. Sulfate ion is to be removed from 60 L of water by exchanging it with chloride ion on 1 L of a strong-base resin with relative molar selectivities as listed in Table 15.6 and an ion-exchange capacity of 1.2 eq/L of resin. The water to be treated has a sulfate-ion concentration of 0.018eq/L and a chloride-ion concentration of 0.002eq/L. Following the attainment of equilibrium ion exchange, the treated water will be removed and the resin will be regenerated with 30 L of 10 wt% aqueous NaCl.
(a) Write the ion-exchange reaction.
(b) Determine the value of KSO2 –4, Cl–.
(c) Calculate equilibrium concentrations cSO2 –4, c,Cl– , q SO2 –4, and qCl– in eqL for the initial ion-exchange step.
(d) Calculate the concentration of C1– in eq/L for the regenerating solution.
(e) Calculate cSO2 –4, cCl–, q SO2 –4, and qCl- upon reaching equilibrium in the regeneration step.
(f) Are the separations sufficiently selective?
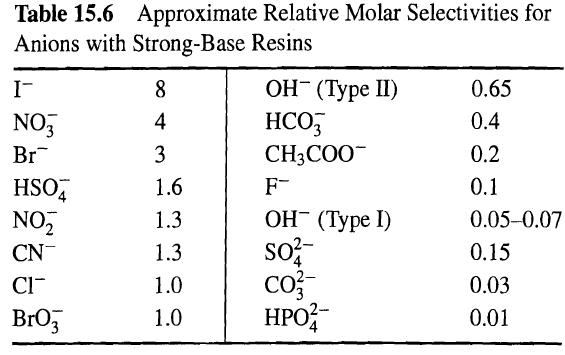
Table 15.6 Approximate Relative Molar Selectivities for Anions with Strong-Base Resins ( I) 0.65 , 0.4 NO, 4 3 CH3COO- 0.2 Br 1.6 0.1 HSO, NO, F- ( I) so? co ? 0.05-0.07 1.3 0.15 CN- 1.3 CI- 1.0 0.03 3 1.0 0.01
Step by Step Solution
3.29 Rating (173 Votes )
There are 3 Steps involved in it
Let ASO 4 and BCl This a case of unequal charges so use Eq 1537 for the reaction a Thus n 2 and by t... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (526).docx
120 KBs Word File


