Determine the diameter and packed height of a countercurrently operated packed tower required to recover 99% of
Question:
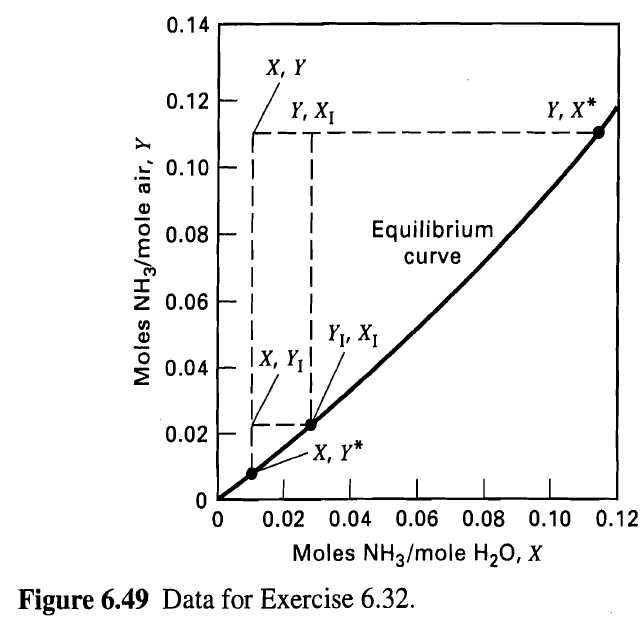
Determine the diameter and packed height of a countercurrently operated packed tower required to recover 99% of the ammonia from a gas mixture that contains 6 mol% NH3 in air. The tower, packed with l-in. metal Pall rings, must handle 2,000 ft3/min of gas as measured at 68oF and 1 atm. The entering water-absorbent rate will be twice the theoretical minimum, and the gas velocity will be such that it is 50% of the flooding velocity. Assume isothermal operation at 68°F and 1 atm. Equilibrium data are given in Figure 6.49
Transcribed Image Text:
0.14 X, Y 0.12 Y, X* Y, X1 0.10 | Equilibrium 0.08 curve 0.06 EI Y1, X1 |X, Y¡! 0.04 0.02 -х, ү* 0.02 0.04 0.06 0.08 0.10 0.12 Moles NH3/mole H20, X Figure 6.49 Data for Exercise 6.32. Moles NH3/mole air, Y
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
Entering gas rate is Compute material balance NH 3 in entering gas 006312 1872 lbmolh NH 3 in exiting gas 0011872 019 lbmolh Air in entering and exiti...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
A rigid 35 ft3 tank contains water initially at 250 F, with 50 % liquid and 50% vapor, by volume. A pressure-relief valve on the top of the tank is set to 150 lbf/in 2 (the tank pressure cannot...
-
A mixture of N2, H2 and NH3 is at equilibrium according to the equation N2(g) + 3H2(g) 2NH3(g) as depicted below. The volume is suddenly decreased (by increasing the external pressure), and a new...
-
A rigid tank contains 1 lb mol of argon gas at 400 R and 750 psia. A valve is now opened, and 3 lb mol of N2 gas is allowed to enter the tank at 340 R and 1200 psia. The final mixture temperature is...
-
Name four ways to mitigate the incentives for managers to overproduce.
-
Distinguish between the journal entries in a periodic inventory system and a perpetual inventory system (a) Made by the buyer, (b) Made by the seller.
-
A 48-in. boom is held by a ball-and-socket joint at C and by two cables BF and DAE; cable DAE passes around a frictionless pulley at A. For the loading shown, determine the tension in each cable and...
-
At a blood drive, 5 donors with type O+ blood, 7 donors with type A+ blood, and 3 donors with type B+ blood are in line. In how many distinguishable ways can the donors be in line?
-
The following events apply to The Ice Cream Parlor for the 2013 fiscal year: 1. The company started when it acquired $20,000 cash from the issue of common stock. 2. Purchased a new ice cream machine...
-
You are flying your glider around your local airport for fun one late Saturday afternoon in July. The convective currents are great that day. You fly for about 1.5 hours enjoying a perfect day for...
-
At the completion of construction, a project's final construction loan balance was US$1000. This amount must be amortized over the term of a 15-year loan with an annual fixed interest rate of 6.85%....
-
You are asked to design a packed column to recover acetone from air continuously, by absorption with water at 60F. The air contains 3 mol% acetone, and a 97% recovery is desired. The gas flow rate is...
-
A tower, packed with Montz B1-200 metal structured packing, is to be designed to absorb SO2 from air by scrubbing with water. The entering gas, at an SO2-free flow rate of 6.90 lbmollh-ft2 of bed...
-
Methamphetamine (meth) is an addictive, synthetic drug made chiefly in small toxic labs (STLs) in homes, tents, barns, or hotel rooms. The manufacturing process is dangerous, often resulting in...
-
Consider the following argument and explain whether or not it is valid, and why: Recycling promotes thorough collection and separation of garbage, so it should be implemented as a means of reducing...
-
Summarize what could be done in order to prevent climate change.
-
Find any legal document, for example, one of the many privacy or user agreements you click through to use apps. Copy the original text, and then revise it for clarity and conciseness. By how many...
-
To discourage people from signing up at the Promise/Cash Center site shown in Figure 18, write an article for a blog that warns consumers about questionable business practices. Your objective is to...
-
How would you describe your typical writing style? Now that you learned guidelines for business writing, how willing are you to flex your style to meet the needs of your audience? In what ways is it...
-
In what sequence should a corporations assets be distributed on liquidation of the corporation?
-
Why is the national security argument for tariffs questionable?
-
The basic idea behind the pseudoscientific subliminal selfhelp industry is that you can change some aspect of your behavior if you let your unconscious process motivational messages (you can lose...
-
A total of 6,000 lb/h of a liquid solution of 40 wt% benzene in naphthalene at 50 C is cooled to 15 C. Use Figure 4.22 to obtain the weight of crystals and the flow rate and composition of mother...
-
A mixture of chloroform (CHCl 3 ) and acetic acid at 18 C and 1 atm (101.3 kPa) is extracted with water to recover the acid. Fortyfive kg of 35 wt% CHCl3 and 65 wt% acid is treated with 22.75 kg of...
-
In a rigorous vaporliquidliquid equilibrium calculation (the so-called three-phase flash), is it necessary to consider all possible phase conditions, i.e., all-liquid, all-vapor, vaporliquid, liquid...
-
123 Anna purchased 100 shares of Delta stock on February 1, Year 2, for $46 per share, and 5 received a two-for-one stock split on December 31, Year 2. Anna sold all the shares of Delta stock on...
-
work i Saved QS 17-10 (Algo) Computing activity rates for activity-based costing LO P3 A company sells two types of products: standard and deluxe. It prepares the following analysis showing budgeted...
-
! Required information [The following information applies to the questions displayed below.] Sweeten Company had no jobs in progress at the beginning of the year and no beginning inventories. It...

Study smarter with the SolutionInn App


