Question: Equation of change for entropy this problem is an introduction to the thermodynamics of irreversible processes. A treatment of multi component mixtures is given in
Equation of change for entropy this problem is an introduction to the thermodynamics of irreversible processes. A treatment of multi component mixtures is given in SS24.1 and 2.
(a) Write an entropy balance for the fixed volume element ∆x ∆y ∆z. Let s be the entropy flux vector, measured with respect to the fluid velocity vector v. Further, let the rate of entropy production per unit volume be designated by gS. Show that when the volume element ∆x ∆y ∆z is allowed to become vanishingly small, one finally obtains an equation of change for entropy in either of the following two forms: in which S is the entropy per unit mass.
(b) If one assumes that the thermodynamic quantities can be defined locally in a non-equilibrium situation, then U can be related to S and V according to the thermodynamic relation dU = TdS - pdV. Combine this relation with Eq. 11.2-2 to get
(c) The local entropy flux is equal to the local energy flux divided by the local temperature12-15 that is, s = q/T. Once this relation between s and q is recognized, we can compare Eqs. 11D.1-2 and 3 to get the following expression for the rate of entropy production per unit volume: The first term on the right side is the rate of entropy production associated with heat transport, and the second is the rate of entropy production resulting from momentum transport. Equation 11D.1-4 is the starting point for the thermodynamic study of the irreversible processes in a pure fluid.
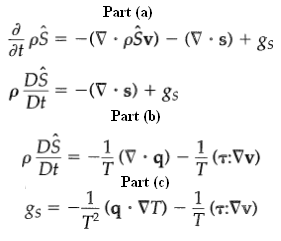
Part (a) , S-7.pS)-(7.s) + Ss at DS -(V s) + 8s Dt Part (b) DS -(V q) (r:Vv) Dt Part (c) (q VT) (7:Vv) 8s T2 (AA:4) - (LA b) -
Step by Step Solution
3.29 Rating (175 Votes )
There are 3 Steps involved in it
Equation of change for entropy a For the volume element AxAyAz we write a balance equation n... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
6-E-C-E-T-P (210).docx
120 KBs Word File


