Question: Ethers undergo an acid-catalyzed cleavage reaction when treated with the Lewis acid BBr3 at room temperature. Propose a mechanism for thereaction. + CHBr CH 1.
Ethers undergo an acid-catalyzed cleavage reaction when treated with the Lewis acid BBr3 at room temperature. Propose a mechanism for thereaction.
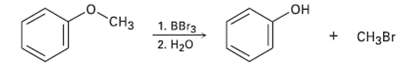
+ CHBr CH 1. r 2. H20
Step by Step Solution
3.51 Rating (164 Votes )
There are 3 Steps involved in it
BBr3 CH3 BBr forms a Lewis acid complex ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-E (83).docx
120 KBs Word File


