Question: Explain why one of these carbocations is much more stable than the other: + CH-CH3 CH CH
Explain why one of these carbocations is much more stable than the other:
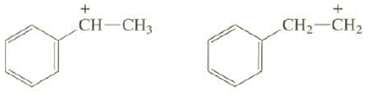
+ CH-CH3 CH CH
Step by Step Solution
★★★★★
3.40 Rating (163 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The p orbital of the positively charged carbon of the carbocation on the left is con... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock
Document Format (1 attachment)
15-C-O-C (121).docx
120 KBs Word File


