Question: Figure shows a cycle consisting of five paths: AB is isothermal at 300 K, BC is adiabatic with work = 5.0 J, CD is at
Figure shows a cycle consisting of five paths: AB is isothermal at 300 K, BC is adiabatic with work = 5.0 J, CD is at a constant pressure of 5 atm, D E is isothermal, and EA is adiabatic with a change in internal energy of 8.0 J. What is the change in internal energy of the gas along pathCD?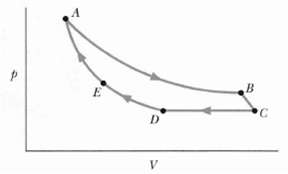
D. V
Step by Step Solution
3.38 Rating (167 Votes )
There are 3 Steps involved in it
For convenience the int subscript for the internal energy will be omitted in this solution R... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
2-P-T-K-T (301).docx
120 KBs Word File


