Question: Figure shows a reversible cycle through which 1.00 mol of a monatomic ideal gas is taken. Assume that p = 2p0, V = 2V0, p0
Figure shows a reversible cycle through which 1.00 mol of a monatomic ideal gas is taken. Assume that p = 2p0, V = 2V0, p0 = 1.01 x 105 Pa, and V0 = 0.0225 m3. Calculate
(a) The work done during the cycle,
(b) The energy added as heat during stroke abc and
(c) The efficiency of the cycle.
(d) What is the efficiency of a Carnot engine operating between the highest and lowest temperatures that occur in the cycle?
(e) Is this greater than or less than the efficiency calculated in(c)
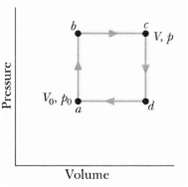
V, P Vo Po Volume Pressure
Step by Step Solution
3.54 Rating (171 Votes )
There are 3 Steps involved in it
a The net work done is the rectangular area enclosed in the pV diagram WVV PPo 2V V 2 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
2-P-T-S-L (234).docx
120 KBs Word File


