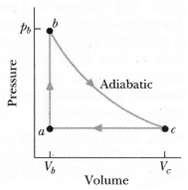
Question: Figure shows a reversible cycle through which 1.00 mol of a monatomic ideal gas is taken. Volume Vc = 8.00%. Process bc is an adiabatic
Figure shows a reversible cycle through which 1.00 mol of a monatomic ideal gas is taken. Volume Vc = 8.00%. Process bc is an adiabatic expansion, with pb = 10.0 atm and Vb = 1.00 x 10-3 m3. For the cycle, find
(a) The energy added to the gas as heat,
(b) The energy leaving the gas as heat,
(c) The net work done by the gas, and
(d) The efficiency of the cycle.
Adiabatic V, V. Volume Pressure
Step by Step Solution
3.36 Rating (177 Votes )
There are 3 Steps involved in it
a Energy is added as heat during the portion of the process from a to b This portion occurs ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
2-P-T-S-L (232).docx
120 KBs Word File


