Question: How might the following compounds be prepared using Michael reactions? Show (he nucleophilic donor and the electrophilic acceptor in eachcase. , , (b) . (a)
How might the following compounds be prepared using Michael reactions? Show (he nucleophilic donor and the electrophilic acceptor in eachcase.
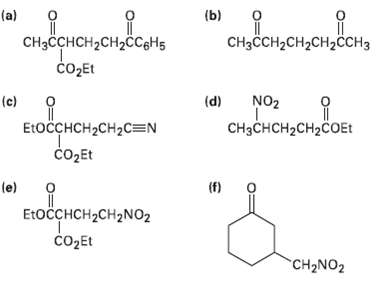
", , (b) . (a) CHCCH-CH2CH2CH3 CH2H2CCgHs o2Et NO2 (d) (c) E:OCCHCH2CH2C=N CHH2H2cOEt Et () (e) EtoCH2CH2NO2 COEL CH2NO2
Step by Step Solution
3.32 Rating (164 Votes )
There are 3 Steps involved in it
Michael reactions occur between stabilized enolate anions and ounsaturated carbonyl compounds Learn ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-C-R (42).docx
120 KBs Word File


