Question: In contrast to the rapid reaction shown in Problem 23.40, ethyl acetoacetate requires a temperature over 150 ?C to undergo the same kind of cleavage
In contrast to the rapid reaction shown in Problem 23.40, ethyl acetoacetate requires a temperature over 150 ?C to undergo the same kind of cleavage reaction. How can you explain the difference in reactivity?
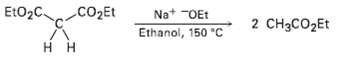
EtO2C CO2Et Na* "OEt Ethanol, 150 C 2 CH3CO2Et H H
Step by Step Solution
3.35 Rating (170 Votes )
There are 3 Steps involved in it
Two different reactions are possible when ethyl acetoacetate reacts with etho... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-C-R (41).docx
120 KBs Word File


