Question: If methanol rather than water is added at the end of a Hell-Volhard-Zelinskii reaction, an ester rather than an acid is produced. Show how you
If methanol rather than water is added at the end of a Hell-Volhard-Zelinskii reaction, an ester rather than an acid is produced. Show how you could carry out the following transformation, and propose a mechanism for the ester-formingstep.
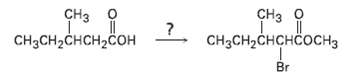
CH CH HCH-CHCHCOH3 CH3CH-CHCH-CO Br
Step by Step Solution
3.27 Rating (176 Votes )
There are 3 Steps involved in it
OH H CHCH3CHCH3 0 PBr3 Br formation of acid bromide HCH3 H Br Br H CHCH3CHCH3 enoliz... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-CA (175).docx
120 KBs Word File


