Question: In a set of experiments on a hypothetical one-electron atom, you measure the wavelengths of the photons emitted from transitions ending in the ground state
In a set of experiments on a hypothetical one-electron atom, you measure the wavelengths of the photons emitted from transitions ending in the ground state (n = I), as shown in the energy level diagram in Fig. 38.37. You also observe that it takes 17.50eV to ionize this atom.(a) What is the energy of the atom in each of the levels (n = I, n = 2, etc.) shown in the figure?(b) If an electron made a transition from the n = 4 to the n = 2 level what wavelength of light would itemit?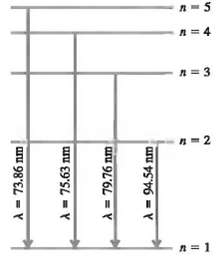
n=1 A = 73.86 nm A = 75.63 nm A = 79.76 nm A = 94.54 nm 3 A=4 8-5
Step by Step Solution
3.29 Rating (184 Votes )
There are 3 Steps involved in it
IDENTIFY and SET UP The wavelength of the photon is related to the ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
P-M-P-P-E-A (19).docx
120 KBs Word File


