Question: In Section 2.6 it was noted that the net bonding energy EN between two isolated positive and negative ions is a function of interionic distance
In Section 2.6 it was noted that the net bonding energy EN between two isolated positive and negative ions is a function of interionic distance r as follows:
where A, B, and n are constants for the particular ion pair. Equation 6.25 is also valid for the bonding energy between adjacent ions in solid materials. The modulus of elasticity E is proportional to the slope of the interionic force–separation curve at the equilibrium interionic separation; that is,
Derive an expression for the dependence of the modulus of elasticity on these A, B, and n parameters (for the two-ion system) using the following procedure:
1. Establish a relationship for the force F as a function of r, realizing that is
2. Now take the derivative dF/dr.
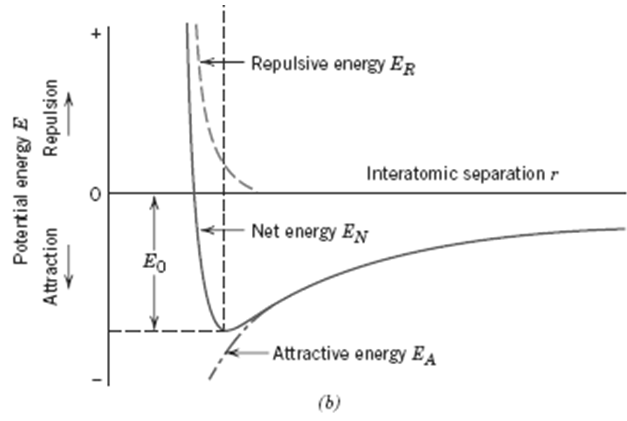
3. Develop an expression for r0, the equilibrium separation. Since r0 corresponds to the value of r at the minimum of the EN-versus-r curve (Figure), take the derivative dEN/dr, set it equal to zero, and solve for r, which corresponds to r0.
4. Finally, substitute this expression for r0 into the relationship obtained by takingdF/dr.
EN %3D (6.25) dF Ecc dr
Step by Step Solution
3.36 Rating (162 Votes )
There are 3 Steps involved in it
This problem asks that we derive an expression for the dependence of the ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (175).docx
120 KBs Word File


