Iodine azide, IN 3 , adds to alkenes by an electrophilic mechanism similar to that of bromine.
Question:
Iodine azide, IN3, adds to alkenes by an electrophilic mechanism similar to that of bromine. If a mono-substituted alkene such as 1-butene is used, only one product results:
(a) Add lone-pair electrons to the structure shown for IN3, and draw a second resonance form for the molecule.
(b) Calculate formal charges for the atoms in both resonance structures you drew for IN3 in part (a).
(c) In light of the result observed when IN3 adds to 1-butene, what is the polarity of the 1?N3 bond? Propose a mechanism for the reaction using curved arrows to show the electron flow in each step.
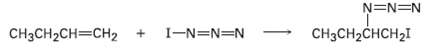
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





