Question: Iodine reacts with mesitylene to form a complex with an absorption maximum at 332 nm in CCl 4 solution: (a) Given that the product absorbs
Iodine reacts with mesitylene to form a complex with an absorption maximum at 332 nm in CCl4 solution:
![[complex] I, + K = [I][mesitylene] Iodine Mesitylene Complex Amax = 332 nm E332 E332 0](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1591/8/1/3/1625ee1242a369801591813157745.jpg)
(a) Given that the product absorbs at 332 nm, but neither reactant has significant absorbance at this wavelength, use the equilibrium constant, K, and Beer's law to show that
![[complex] I, + K = [I][mesitylene] Iodine Mesitylene Complex Amax = 332](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1591/8/1/3/2005ee12450de4f11591813195752.jpg)
where A is the absorbance at 332 nm, ε is the molar absorptivity of the complex at 332 nm, [mesitylene] is the concentration of free mesitylene, and [I2]tot is the total concentration of iodine in the solution (= [I2] + [complex]). The cell pathlength is 1.000 cm.
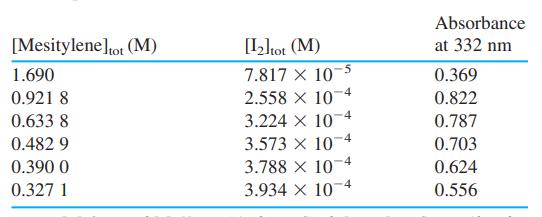
(b) Spectrophotometric data for this reaction are shown in the table. Because [mesitylene]tot >> [I2], we can say that [mesitylene] [mesitylene]tot. Prepare a graph of A/([mesitylene][I2]tot) versus A/[I2]tot and find the equilibrium constant and molar absorptivity of the complex.
[complex] I, + K = [I][mesitylene] Iodine Mesitylene Complex Amax = 332 nm E332 E332 0
Step by Step Solution
3.53 Rating (174 Votes )
There are 3 Steps involved in it
We will make the substitutions complex A and I 2 l 2 t... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
878-E-C-E-E-C (2166).docx
120 KBs Word File


