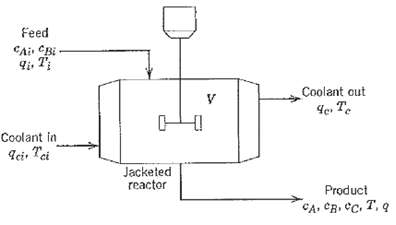
Question: Irreversible consecutive reactions A ? B ? C occur in a jacketed, stirred-tank reactor as shown in Figure. Derive a dynamic model based on the
Irreversible consecutive reactions A ? B ? C occur in a jacketed, stirred-tank reactor as shown in Figure. Derive a dynamic model based on the following assumptions: (i) The contents of the tank and cooling jacket are well mixed. The volumes of material In the jacket arid in the tank do not vary with time. (ii) The reaction rates are given by
![]()
(iii) The thermal capacitances of the tank contents and the jacket contents are significant relative to the thermal capacitances of the jacket and tank walls, which can be neglected. (iv) Constant physical properties and heat transfer coefficients can be assumed. Note: All flow rates arc volumetric flow rates in L/h. The concentrations have units of mol/L. The heats of reaction are ?H1 and ?H2.
7n = kie-EiRTCA(=] mol A/h L 72 = kze-FaRTCA [-] mol B/h L
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
Assume that the feed contains only A and B and no C Component balances fo... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
38-E-C-E-P-C (18).docx
120 KBs Word File


