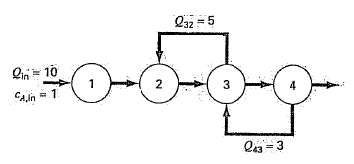
Question: An irreversible, first-order reaction (see Sec. 28.1) takes place in four well-mixed reactors (figure), Thus, the rate at which A is transformed to B can
An irreversible, first-order reaction (see Sec. 28.1) takes place in four well-mixed reactors (figure),
Thus, the rate at which A is transformed to B can be represented asRαb = k VcThe reactors have different volumes, and because they are operated at different temperatures, each has a different reaction rate:

Determine the concentration of A and B in each of the reactors at steadystate.
A B
Step by Step Solution
3.40 Rating (163 Votes )
There are 3 Steps involved in it
Steadystate mass balances for A in each reactor can be written as Stead... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
45-M-N-A-N-L-A (104).docx
120 KBs Word File


