Question: Ketones react with alcohols to yield products called acetals. Why does the allcis isomer of 4-tert-butyl-l, 3-cyclohexanediol react readily with acetone and an acid catalyst
Ketones react with alcohols to yield products called acetals. Why does the allcis isomer of 4-tert-butyl-l, 3-cyclohexanediol react readily with acetone and an acid catalyst to form an acetal while other stercoisomers do not react? In formulating your answer, draw the more stable chair conformations of all four stereoisomers and the product acetal. Use molecular models for help.
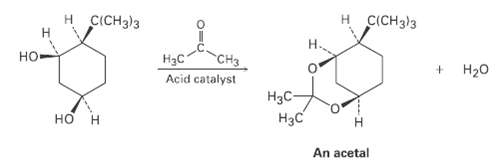
H CICH3)3 CICH)3 H3C CH3 Acid catalyst H20 H3C- An acetal
Step by Step Solution
3.32 Rating (158 Votes )
There are 3 Steps involved in it
Draw the four possible isomers of 4tertbutylcyclohexane13diol Make models of these isomers als... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-O-C (161).docx
120 KBs Word File


