Measurement of diffusivity by unsteady-state evaporation, use the following data to determine the diffusivity of ethyl propionate
Question:
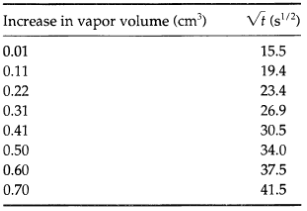
Measurement of diffusivity by unsteady-state evaporation, use the following data to determine the diffusivity of ethyl propionate (species A) into a mixture of 20 mole% air and 80 mole% hydrogen (this mixture being treated as a pure gas B)
These data were obtained by using a glass tube 200 cm long, with an inside diameter 1.043 cm; the temperature was 27.9°C and the pressure 761.2 mm Hg. The vapor pressure of ethyl propionate at this temperature is 41.5 mm Hg. Note that t is the actual time from the start of the evaporation, whereas the volume increase is measured from t ≈ 240 s.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





