Reduction of evaporation losses by transpiration (Fig. 11B.7), it is proposed to reduce the rate of evaporation
Question:
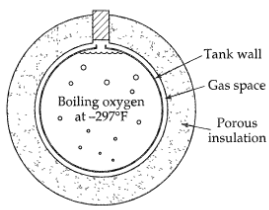
Reduction of evaporation losses by transpiration (Fig. 11B.7), it is proposed to reduce the rate of evaporation of liquefied oxygen in small containers by taking advantage of transpiration. To do this, the liquid is to be stored in a spherical container surrounded by a spherical shell of a porous insulating material as shown in the figure. A thin space is to be left between the container and insulation, and the opening in the insulation is to be stoppered. In operation, the evaporating oxygen is to leave the container proper, move through the gas space, and then flow uniformly out through the porous insulation. Calculate the rate of heat gain and evaporation loss from a tank 1 ft in diameter covered with a shell of insulation 6 in. thick under the following conditions with and without transpiration.
Temperature of liquid oxygen.....................................– 297°F
Temperature of outer surface of insulation..............30°F
Effective thermal conductivity of insulation..............0.02 Btu/hr ∙ ft ∙ F
Heat of eyaporation of oxygen...................................91.7 Btu/lb
Average Cp of 02 flowing through insulation............0.22 Btu/lb ∙ F
Neglect the thermal resistance of the liquid oxygen, container wall, and gas space, and neglect heat losses through the stopper. Assume the particles of insulation to be in local thermal equilibrium with the gas.
Step by Step Answer:






