Question: Samples A and B are at different initial temperatures when they are placed in a thermally insulated container and allowed to come to thermal equilibrium.
Samples A and B are at different initial temperatures when they are placed in a thermally insulated container and allowed to come to thermal equilibrium. Figure a gives their temperatures Z versus time t. Sample A has a mass of 5.0 kg; sample B has a mass of 1.5 kg. Figure b is a general plot for the material of sample B. It shows the temperature change AZ that the material undergoes when energy is transferred to it as heat Q. The change ?T is plotted versus the energy Q per unit mass of the material, and the scale of the vertical axis is set by ?Ts = 4.0 Co. What is the specific heat of sample A?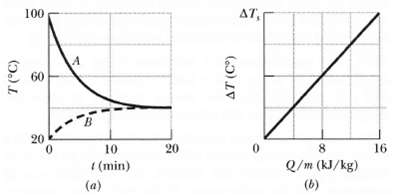
. 100 A. 60 20 8. Q/m (kJ/kg) 10 20 16 1 (min) (6) (a) (3.)L AT (C)
Step by Step Solution
3.36 Rating (159 Votes )
There are 3 Steps involved in it
We note that the heat capacity of sample B is given by the reciprocal of the slope of the ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
2-P-T-T (337).docx
120 KBs Word File


