Selenium from 0.108 g of Brazil nuts was converted into the fluorescent product in Reaction 17-15, which
Question:
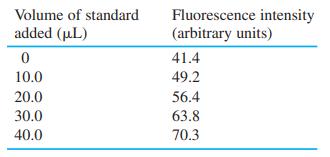
Selenium from 0.108 g of Brazil nuts was converted into the fluorescent product in Reaction 17-15, which was extracted into 10.0 mL of cyclohexane. Then 2.00 mL of the cyclohexane solution were placed in a cuvet for fluorescence measurement. Standard additions of fluorescent product containing 1.40 g Se/mL are given in the table. Construct a standard addition graph to find the concentration of Se in the 2.00-mL unknown solution. Find the wt% of Se in the nuts and its uncertainty and 95% confidence interval.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





