Question: Shown here are some pKa data for simple dibasic acids. How can you account for the fact that the difference between the first and second
Shown here are some pKa data for simple dibasic acids. How can you account for the fact that the difference between the first and second ionization constants decreases with increasing distance between the carboxyl groups?
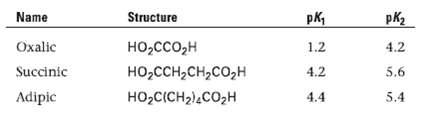
Name Structure pk, pK2 Oxalic Succinic 4.2 1.2 HO,cco,H --,, -ICH2),c,H 4.2 5.6 4.4 Adipic 5.4
Step by Step Solution
3.40 Rating (163 Votes )
There are 3 Steps involved in it
Inductive effects of functional groups are transmitted through bonds For oxalic acid the e... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-CA (70).docx
120 KBs Word File


