Question: Solve Exercise 4.19 by assuming an ideal solution and using vapor pressure data from Figure. Also determine the temperature. 10,000 6,000 4,000 2,000 | o
Solve Exercise 4.19 by assuming an ideal solution and using vapor pressure data from Figure. Also determine the temperature.
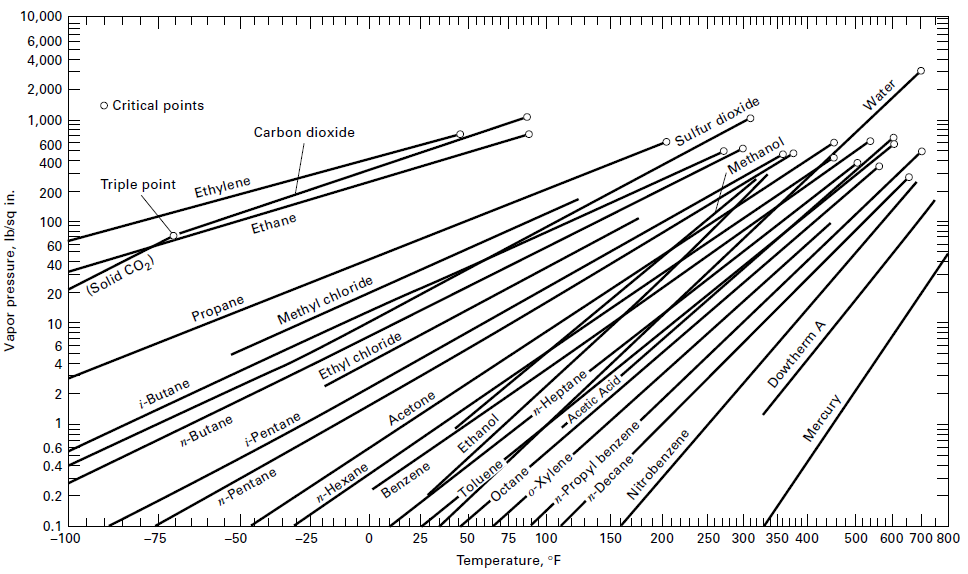
10,000 6,000 4,000 2,000 | o Critical points 1,000 600 400 Carbon dioxide 200 Triple point T 100 Ethylene 60 40 Ethane Sulfur dioxide Water 20 (Solid CO2) Methanol 10 Propane 4 Methyl chloride i-Butane Ethyl chloride 0.6 0.4 n-Butane i-Pentane 0.2 Acetone n-Hepta ne 0.1 -100 -Aceic Acid- n-Pentane Ethanol Octane -o-Xylene- n-Propyl benzene -e -75 KIn ulu Toluene: Benzene -50 -25 25 75 100 Temperature, E 150 200 250 300 350 400 500 600 700 800 Vapor pressure, Ib/sqir n-Decane Nitrobenzene Dowtherm A Mercury
Step by Step Solution
3.38 Rating (176 Votes )
There are 3 Steps involved in it
Basis F 100 mole with 60 moles B and 40 moles A Want 0960 54 moles B in liquid Therefore 60 54 6 mol... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (109).docx
120 KBs Word File


