Question: Using vapor pressure data from Exercises 4.6 and 4.8 and the enthalpy data provided below: (a) Construct an h-x-y diagram for the benzene-toluene system at
Using vapor pressure data from Exercises 4.6 and 4.8 and the enthalpy data provided below:
(a) Construct an h-x-y diagram for the benzene-toluene system at 1 atm (101.3 kPa) based on the use of Raoult's and Dalton's laws.
(b) Calculate the energy required for 50 mol% vaporization of a 30 mol% liquid solution of benzene in toluene, initially at saturation temperature. If the vapor is then condensed, what is the heat load on the condenser in kJ/kg of solution if the condensate is saturated and if it is subcooled by10?C?
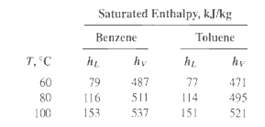
Saturated Enthalpy, kJkg Benzene Toluene T, C hy hi hy 79 487 77 471 60 80 116 511 114 495 100 153 537 151 521
Step by Step Solution
3.43 Rating (169 Votes )
There are 3 Steps involved in it
a First compute the vapor and liquid equilibrium compositions at 1 atm and temperatures from 60 to 100 o C using Raoults law with the vapor pressure d... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (101).docx
120 KBs Word File


