The last stage of a liquefaction process is shown in diagrammatic form in Fig. P18.2. Derive the
Question:
The last stage of a liquefaction process is shown in diagrammatic form in Fig. P18.2. Derive the relationship between \(p_{1}\) and \(T_{1}\) for the maximum yield of liquid at conditions \(p_{L}, T_{L}, h_{L}\) for a gas obeying the state equation
\[\left(p+\frac{1.368}{v_{m}^{2}}\right)\left(v_{m}-0.0367\right)=\Re T\]
where \(p\) is pressure (bar), \(v_{m}\) is the molar volume \(\left(\mathrm{m}^{3} / \mathrm{kmol}\right)\), and \(T\) is temperature \((\mathrm{K})\).
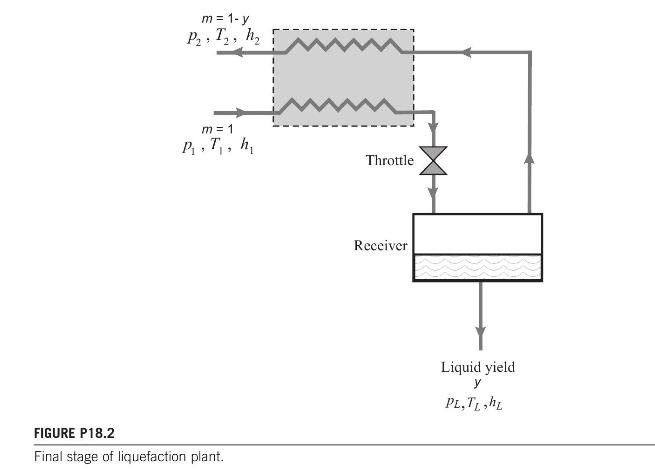
Calculate the pressure for maximum yield at a temperature of \(120 \mathrm{~K}\).
\[\left[p=\frac{a}{b^{2}}\left(1-\sqrt{\frac{\Re b T_{\mathrm{i}}}{2 a}}\right)\left(3 \sqrt{\frac{\Re b T_{\mathrm{i}}}{2 a}}-1\right) ; 62.8 \text { bar }\right]\]
Step by Step Answer:

Advanced Thermodynamics For Engineers
ISBN: 9780080999838
2nd Edition
Authors: D. E. Winterbone, Ali Turan





