An exothermic, liquid-phase reaction is to take place in a stirred tank reactor. The feed to the
Question:
An exothermic, liquid-phase reaction is to take place in a stirred tank reactor. The feed to the reactor (F) is pure A at 500 mol/min with a concentration of 10 mol/L. The volume of the reactor is 100 L, and the feed temperature is 30°C. A reaction takes place in which A is converted to B (A ® B) by a first-order reaction:![1650 -TA = KCA = 3e TK CA[mol/m/s]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/4/3/9/528654b63a8ae7501699439526952.jpg)
The heat of reaction (−ΔH ) is 75,240 J/mol, and it is approximately constant from 30°C to 200°C. Other data for the process and coolant are as follows: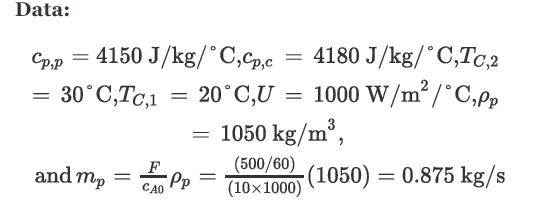
For this system, calculate the following:
1. If the reactor is to be run adiabatically (no coolant), at what temperature and conversion will the reactor run?
2. If it is desired to obtain 80% conversion, at what temperature must the reaction run?
3. For the case in Part (b), what must be the flow of coolant fed to the cooling coil in the reactor?
4. What heat transfer area is required for Part (c)?
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





