Consider the following process in which liquid feed material A (normal BP of 110C) is reacted with
Question:
Consider the following process in which liquid feed material A (normal BP of 110°C) is reacted with gaseous feed material G to produce main product C and byproducts R and S via the following reactions: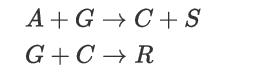
Both feeds enter the process at ambient temperature and pressure. Both reactions occur in the gas phase at moderate temperature and pressure (250°C and 10 bar). The normal boiling points of G, S, and C are less than −120°C. Byproduct R has a normal boiling point of 75°C and is highly soluble in water. Product C is very soluble in water but G and S are insoluble. The single-pass conversion through the reactor is low for feed A, and the ratio of G to A in the feed to the reactor should be maintained in excess of 4 to minimize the chance of other unwanted side reactions. Using this information, and assuming that both A and G are expensive, do the following:
1. Draw a preliminary process flow diagram identifying the main unit operations (reactors, compressors, pumps, heat exchangers, and separators), and identify the recycle structure of the process.
2. Justify the methods used to recycle A and G.
3. What unit operations do you suggest for your separators? Justify your choices.
4. How would your PFD change if the price of feed material G were very low?
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting




