The production of ammonia (a key ingredient for fertilizer) using the Haber process takes place at temperatures
Question:
The production of ammonia (a key ingredient for fertilizer) using the Haber process takes place at temperatures of around 500°C and pressures of 250 atm using a porous iron catalyst according the following highly exothermic synthesis reaction: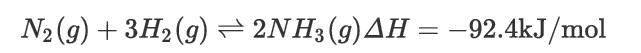
Give possible reasons for the high temperature and pressure used for this reaction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting
Question Posted:





