A biochemical reaction takes place in a 1.00 ml solution of 0.0250 M phosphate buffer initially at
Question:
A biochemical reaction takes place in a 1.00 ml solution of 0.0250 M phosphate buffer initially at pH = 7.20 (see Table 2.6 for pKas of phosphate species).
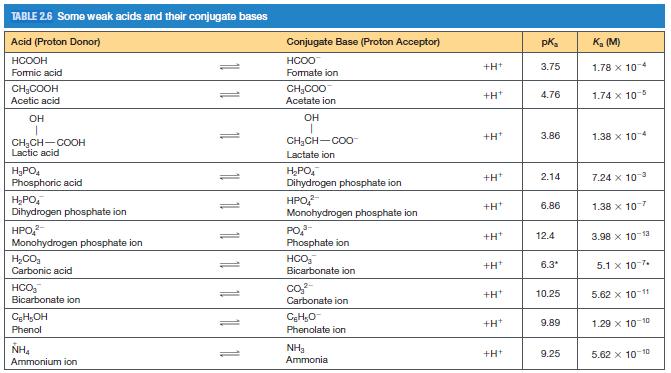
(a) Are the concentrations of any of the four possible phosphate species negligible? If so, identify them and explain your answer.
(b) During the reaction, 3.80 чmol of HCl are produced. Calculate the final pH of the reaction solution. Assume that the HCl is completely neutralized by the buffer.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Biochemistry Concepts And Connections
ISBN: 9780134641621
2nd Edition
Authors: Dean Appling, Spencer Anthony-Cahill, Christopher Mathews
Question Posted:





