The ClausiusClapeyron Law relates the vapor pressure of water P (in atmospheres) to the temperature T (in
Question:
The Clausius–Clapeyron Law relates the vapor pressure of water P (in atmospheres) to the temperature T (in kelvins):
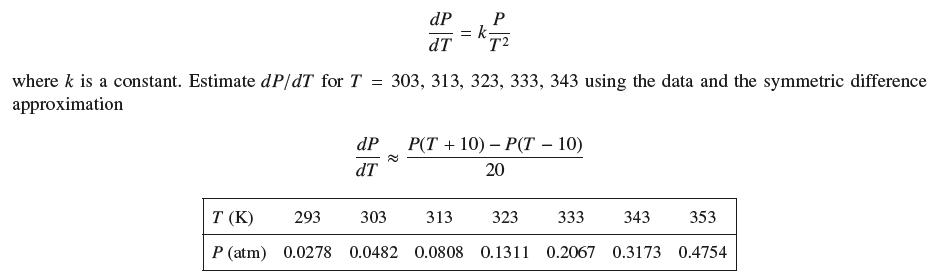
Do your estimates seem to confirm the Clausius–Clapeyron Law? What is the approximate value of k?
Transcribed Image Text:
T (K) P (atm) dP dT dP dT ≈ = k where k is a constant. Estimate dP/dT for T = 303, 313, 323, 333, 343 using the data and the symmetric difference approximation P T2 293 303 313 0.0278 0.0482 0.0808 P(T+10) P(T - 10) - 20 323 333 343 353 0.1311 0.2067 0.3173 0.4754
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Using the indicated approximation to the first derivative we c...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Mathematics questions
-
The vapor pressure of water at temperature T (in kelvins) is the atmospheric pressure P at which no net evaporation takes place. Use the following table to estimate P²(T ) for T = 303, 313, 323,...
-
The Dew Point The vapor pressure of water (see Problem 18.88) decreases as the temperature decreases. If the amount of water vapor in the air is kept constant as the air is cooled, a temperature is...
-
The vapor pressure of water at various temperatures follows: (a) Draw a scatter diagram of these data. What type of relationship seems appropriate in relating y to x? (b) Fit a simple linear...
-
advice on how healthcare leaders can move their organizations toward being an HRO?
-
You are providing financial advice to a shrimp farmer who will be harvesting his last crop of farm-raised shrimp. His current shrimp crop is very young and will, therefore, grow and become more...
-
The nozzle shown in Fig. P3.84 has two water manometers to indicate the static pressures at sections 1 and 2 . The diameters \(D_{1}\) and \(D_{2}\) are 8 in. and 2 in., respectively. Air flows...
-
a. What is meant by the term subsequent events? b. Identify the two types of subsequent events and the criteria used in distinguishing between them.
-
The project information for the custom order project of the Air Control Company is presented here. Draw a project network for this project. Compute the early and late activity times and the slack...
-
if there is a no seasonal effect, about how big, on average, would you expect the x2 statistic to be (what is the mean of the x2 distribution)?
-
A power law model relating the kidney mass K in mammals (in kilograms) to the body mass m (in kilograms) is given by K = 0.007m 0.85 . Calculate dK/dm at m = 68. Then calculate the derivative with...
-
Show, using the limit definition of the derivative, that (x) = |x 2 4| is not differentiable at x = 2.
-
For each of the functions in Exercises 11 through 14, compute the second-order partial derivatives f xx , f yy , f xy , and f yx . f(x, y) = x 2 + y 3 2xy 2
-
Question 2 3. Write simulated conversation where you are talking to Janis about suspected harassment. Your job is to provide evidence that you can use effective communication in the context of an...
-
(1) For multiple linear regression, In our cases, X = ( 2 2 3 ? ? 1 matrix. -1 B = (X'X)X? -1 B = (XX) X ? = X' ? = (2) ? X'X = ? = [1 ] and also ( X'X) = [1] ? ?=1 = ? (? ?=1 ? ? 2 ...... ? 2 (? (?...
-
As part of your analysis, you are required to analyze the company's sources and uses of cash for 2022. Required: Prepare a Cash Flow Statement for Lang Industrial Systems Inc. for year ended 2022. 12...
-
The function f(x, y) = (x + y-11) + (x+y-7) is known as Himmelblau's function which is a function designed for testing per- formance of optimization algorithms (you can see more examples here)....
-
Suppose we have the following two-player game: Player A Top Bottom 3 -17 Left -5 -2 Player B -7 12 A Determine the equilibrium level of production for each firm (4-42). B. Determine the equilibrium...
-
Iggy Company is considering three capital expenditure projects. Relevant data for the projects are as follows. Annual income is constant over the life of the project. Each project is expected to have...
-
Explain the buyers position in a typical negotiation for a business. Explain the sellers position. What tips would you offer a buyer about to begin negotiating the purchase of a business?
-
Use calculus to find the area of the triangle with the given vertices. (0, 0), (3, 1), (1, 2)
-
Evaluate the integral and interpret it as the area of a region. Sketch the region. /2 sin x cos 2x dx
-
Evaluate the integral and interpret it as the area of a region. Sketch the region. /2 sin x cos 2x dx
-
Prove Ther Compute the kernel for the homomorphism : ZZ such that o(1) = 12. is {0}. Let ZZ and o(1) = 12. : Then ker() = {x Z\o(x) = 0}. Since (x)=(1+1+1+...x times) = x(1) and (1) = 12, then (x) =...
-
Suppose everyone believes that only Type A people will withdraw at T=1. In this case the fraction of withdrawers is = 0. The Type A folks receive 1 C* = > 1 0+p(1-0) At the same time the Type B...
-
Find all subgroups of 72 x 7y of order 4 264 26.2 x 24. (0,0) (1,0) Answer: (0,1) (1,1) (0,2) (1,2) {(0,0), (0,1), (0,2), (0, 3)} and (0,3) (1,3) {(1, 0), (1, 1), (1,2), (1,3)} Subgroups of order 4

Study smarter with the SolutionInn App


