Fossil fuels, such as oil, coal and natural gas, all contain some sulfur. When these fuels are
Question:
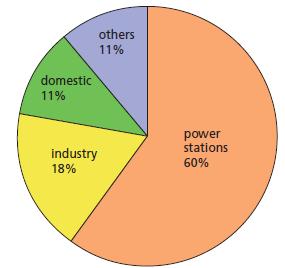
Fossil fuels, such as oil, coal and natural gas, all contain some sulfur. When these fuels are burned they produce many different gases. Concern has grown in recent years about the effects of one of these gases, sulfur dioxide. When sulfur dioxide dissolves in rainwater it forms an acidic solution which has become known as acid rain. Money has been made available to solve the problem of acid rain. Attempts are being made to clean gases being released from power stations and to look into ways in which the effects of acid rain can be reversed. The table below and Figure 12.5 (p. 199) give some data about the emission of sulfur dioxide.
Million tonnes per year
USA ................................................. 26
Russia/Ukraine .............................. 18
Germany .......................................... 7
UK ..................................................... 5
Canada ............................................. 5
France .............................................. 3
Poland ............................................. 3
Italy .................................................. 3
Other countries ............................ 30
a. Using the figures in the table, produce a bar chart to show the amount of sulfur dioxide produced by each of the countries listed.
b. What percentage of the world’s sulfur dioxide is produced in:
(i) France?
(ii) North America?
c. Using the information above, explain why countries such as the US, Russia and Germany are at the top of the list of sulfur dioxide producers.
d. If the total amount of sulfur dioxide produced by Canada is 5 million tonnes per year, what amount is produced by:
(i) Power stations?
(ii) Domestic users?
(iii) Industry?
Figure 12.5
Step by Step Answer:






