a. Explain why energy is needed for boiling even though the temperature of the liquid remains constant.
Question:
a. Explain why energy is needed for boiling even though the temperature of the liquid remains constant.
This diagram shows an apparatus that can be used to measure the specific latent heat of vaporisation of nitrogen.
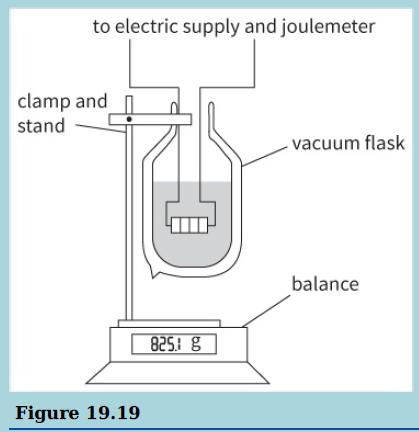
b. Suggest why the nitrogen is contained in a vacuum flask.
c. The change in mass of the nitrogen is measured over a specific time interval with the heater switched off. The heater is switched on, transferring energy at 40 W, and the change of mass is found once more.
The results are shown in the table.
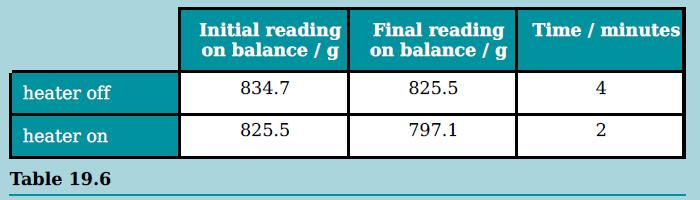
Calculate the specific latent heat of vaporisation of liquid nitrogen.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Physics Coursebook
ISBN: 9781108859035
3rd Edition
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
Question Posted:





