a. i. Explain what is meant by the waveparticle duality of electromagnetic radiation. ii. Explain how the
Question:
a. i. Explain what is meant by the wave–particle duality of electromagnetic radiation.
ii. Explain how the photoelectric effect gives evidence for this phenomenon.
The diagram shows the maximum kinetic energy E of the emitted photoelectrons as the frequency f of the incident radiation on a sodium plate is varied.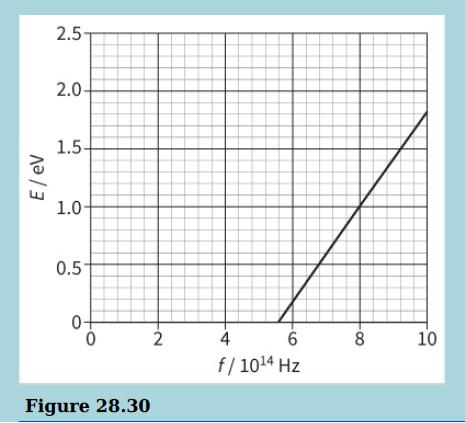
b. Explain why there are no photoelectrons emitted when the frequency of the incident light is less than 5.6 × 1014 Hz.
c. Determine the work function for sodium. Explain your answer.
d. Use the graph to determine the value of the Planck constant. Explain your answer.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Physics Coursebook
ISBN: 9781108859035
3rd Edition
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
Question Posted:





