This question is about the following reaction: 9.20 g of ethanol are mixed with 12.00 g of
Question:
This question is about the following reaction:
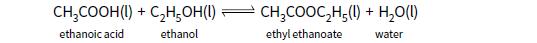
9.20 g of ethanol are mixed with 12.00 g of ethanoic acid in an inert solvent. The total volume of solution is 250 cm3. The mixture is left to equilibrate for several days. At equilibrium 70% of the reactants are converted to products.
a. What is the concentration of each reactant at the start?
b. What is the concentration of each reactant at equilibrium?
c. What is the concentration of each product at equilibrium?
d. i. Write the equilibrium expression for this reaction.
ii. Calculate the value of Kc for the reaction.
iii. Explain why there are no units for Kc for this reaction.
e. What will happen to the numerical value of Kc if 100 cm3 of water is added to the equilibrium mixture?
f. What will happen to the yield of ethyl ethanoate if 100 cm3 of water is added to the equilibrium mixture? Explain your answer.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





