a. Show that b. Use the result of part (a) to show that for a stable system
Question:
a. Show that
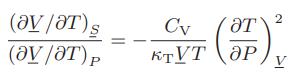
b. Use the result of part (a) to show that for a stable system at equilibrium (∂V /∂T)S and (∂V /∂T)P must have opposite signs.
c. Two separate measurements are to be performed on a gas enclosed in a piston-and-cylinder device. In the first measurement the device is well insulated so there is no flow of heat to or from the gas, and the piston is slowly moved inward, compressing the gas, and its temperature is found to increase. In the second measurement the piston is free to move and the external pressure is constant. A small amount of heat is added to the gas in the cylinder, resulting in the expansion of the gas. Will the temperature of the gas increase or decrease?
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





