Intermolecular forces play an important role in determining the thermodynamic properties of fluids. To see this, consider
Question:
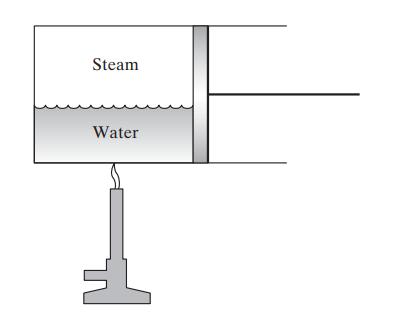
Intermolecular forces play an important role in determining the thermodynamic properties of fluids. To see this, consider the vaporization (boiling) of a liquid such as water in the frictionless piston-and-cylinder device shown.
a. Compute the work obtained from the piston when 1 kg of water is vaporized to steam at 100°C (the vapor and liquid volumes of steam at the boiling point can be found in the steam tables).
b. Show that the heat required for the vaporization of the steam is considerably greater than the work done.
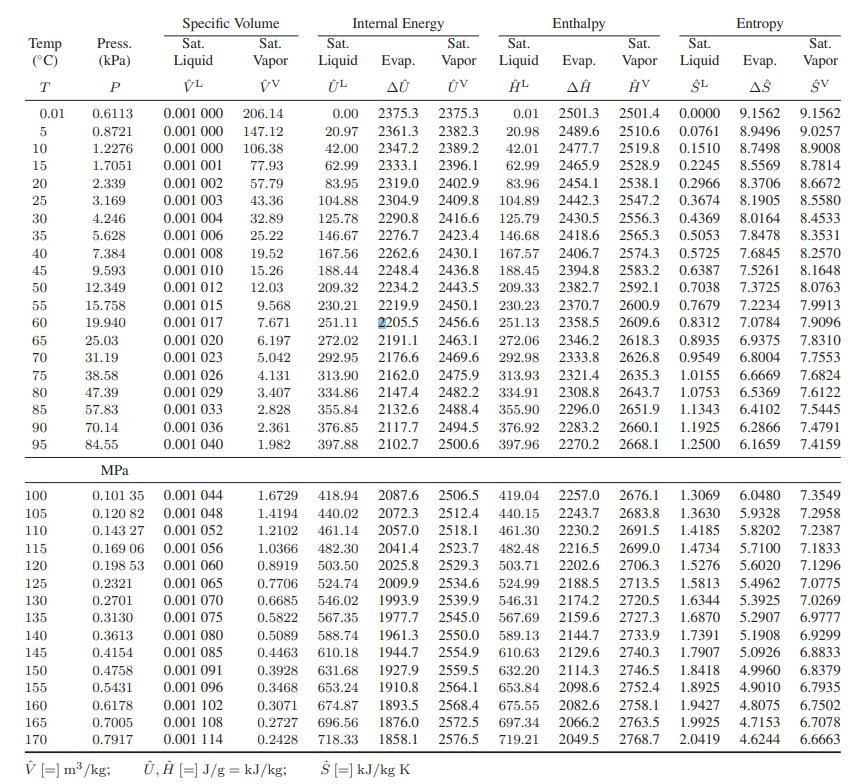
(Note that the enthalpy change for the vaporization is given as 2257 kJ/kg in the steam tables in Appendix A.III.)
Appendix A.III.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





