One of the beauties of thermodynamics is that it provides interrelationships between various state variables and their
Question:
One of the beauties of thermodynamics is that it provides interrelationships between various state variables and their derivatives so that information from one set of experiments can be used to predict the results of a completely different experiment. This is illustrated here.
a. Show that
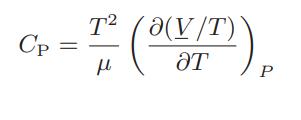
Thus, if the Joule-Thomson coefficient μ and the volumetric equation of state (in analytic or tabular form) are known for a fluid, CP can be computed. Alternatively, if CP and μ are known, (∂(V /T)/∂T)P can be calculated, or if CP and (∂(V /T)/∂T)P are known, μ can be calculated. b. Show that
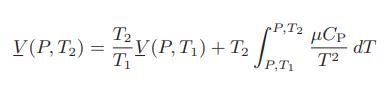
so that if μ and CP are known functions of temperature at pressure P, and V is known at P and T1, the specific volume at P and T2 can be computed.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





