The dissociation pressure of calcium oxalate in the reaction at various temperatures is Compute the standard-state Gibbs
Question:
The dissociation pressure of calcium oxalate in the reaction
![]()
at various temperatures is
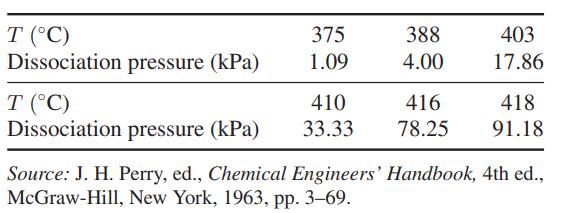
Compute the standard-state Gibbs energy change, enthalpy change, and entropy change for this reaction for the temperature range in the table.
Transcribed Image Text:
CaC₂O4(s) = CaCO3(s) + CO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
The image you sent shows the dissociation pressure of calcium oxalate in the reaction CaC2O4s CaCO3s CO at various temperatures The dissociation press...View the full answer

Answered By

Munibah Munir
I've done MS specialization in finance’s have command on accounting and financial management. Forecasting and Financial Statement Analysis is basic field of my specialization. On many firms I have done real base projects in financial management field special forecasting. I have served more than 500 Clients for more than 800 business projects, and I have got a very high repute in providing highly professional and quality services.I have capability of performing extra-ordinarily well in limited time and at reasonable fee. My clients are guaranteed full satisfaction and I make things easy for them. I am capable of handling complex issues in the mentioned areas and never let my clients down.
4.60+
467+ Reviews
648+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
The first step in biological glycolysis (the catabolic reaction for glucose consumption) involves addition of a phosphate to create glucose 6-phosphate 2 . If the reaction were to occur in aqueous...
-
The average energy output of a good grade of coal is 2.6 10 7 kJ/ton. Fission of 1 mol of 235 U releases 2.1 10 10 kJ. Find the number of tons of coal needed to produce the same energy as 1 lb of...
-
Discriminate the Enablers and the Inhibitors to IT strategy alignment from the below IT fails to meet commitments IT does not understand business IT involved in strategy development IT understands...
-
The following are several independent errors: 1. In January 2007 repair costs of $9,000 were debited to the Machinery account. At the beginning of 2007 the book value of the machinery was $100,000....
-
Why do you think managers underutilize project selection models?
-
Mr. Jenkins, this is typical question: Do you feel that I have treated you fairly in this interview?
-
Weston Corporation manufactures auto parts for two leading Japanese automakers. Nancy Evans is the management accountant for one of Westons largest manufacturing plants. The plants general manager,...
-
An owner arrives to collect her dog's prescription for ampicillin tablets. The dog weighs 23 kg and the dose of ampicillin suggested by the vet is 15 mg/kg/t.i.d. You have the tablets available in...
-
The following data are available for the solubility of barium sulfate in water: where values for the parameter for water are given in Table 9.10-1. a. Compute K s , the ideal solution solubility...
-
Redo Problem 9.22 using Aspen Plus. Problem 9.22 a. Given experimental data either for the excess Gibbs energy, G ex , or for species activity coefficients from which G ex can be computed, it is...
-
A symmetric body moves without the influence of force or torques. Let x3 be the symmetry axis of the body and L be along x3. The angle between w and x3 is a. Let w and L initially be in the x2-x3...
-
4. [Decision Trees] (6 pts) In class, we covered how to learn binary decision trees. In this question, you will modify the decision tree learning algorithm to obtain ternary decision trees (i.e, each...
-
One of the classical paradoxes of Zeno runs (more or less) as follows: A pair of dance partners are two units apart and wish to move together, each moving one unit. But for that to happen, they must...
-
Consider the following decision version of MIN SPANNING TREE MIN SPANNING TREE, DECISION VERSION Input: A graph G = (V, E) with positive edge weights e: E R>0, and a number k N Output: True if G...
-
53. Charle, a seller's agent, sells her client's home for $425,000. Her commission is 6.5%. Charle's total commission is: $267,250 $42,500 $2,762.50 O $27,625 Save and continue Revise question later
-
8. Rewrite the following statements using the symbol V and the variable x. a) Every real number is positive, negative, or zero. b) All students are eager to learn. 9. Rewrite the following statements...
-
Stormy Weather has no attractive investment opportunities. Its return on equity equals the discount rate, which is 10%. Its expected earnings this year are $4 per share. Find the stock price, P/E...
-
The maximum pressure that can be developed for a certain fluid power cylinder is 15.0 MPa. Compute the required diameter for the piston if the cylinder must exert a force of 30 kN.
-
Consider the following variables expressing a football teams strategy. T = It is third down L = We must gain more than 8 yards to get a first down P = We will throw a pass The teams strategy is...
-
Consider the following circuit diagram and the variables L = the light is on, A = switch A is closed, B = switch B is closed, and C = switch C is closed. Write a logic equation for L in terms of A,...
-
Consider the following logic variables for a car: W = the seatbelt warning light is on Ps = there is a passenger in the passenger seat Db = the drivers seat belt is fastened Pb = the passenger seat...
-
Paul has a margin account with a balance of $150,000. The initial margin deposit is 60 percent and Choco Industries is currently selling at $50 per share. a. How many shares of Choco can Paul...
-
Dwayne Security Network Ltd . ( DSN ) is a producer of many alarm systems including, fire alarms, home, and personal security systems. ( DSN ) has already invested $ 1 . 8 million dollars into...
-
On 1 April, you purchased 300 shares of GameStop (GME) at $100 per share on margin. There was a 50% initial margin, which you paid in cash. The price rose to $120 per share, and you closed out your...

Study smarter with the SolutionInn App


