The first step in biological glycolysis (the catabolic reaction for glucose consumption) involves addition of a phosphate
Question:
The first step in biological glycolysis (the catabolic reaction for glucose consumption) involves addition of a phosphate to create glucose 6-phosphate2–. If the reaction were to occur in aqueous solution “chemically” (as compared to “biochemically’”), it would be written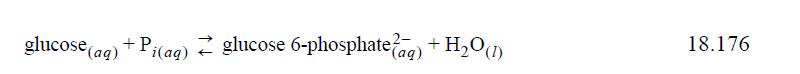
However, in a biological solution, ATP and ADP are carriers of phosphate. Another reaction in solution is the hydrolysis of ATP to ADP:![]()
The transformed values of the Gibbs energies of formation and enthalpy of formation (kJ/mol) at 298.15K, pHc 7.0, I 0.25m, pMg 3.0 are tabulated below along with the physiological concentrations.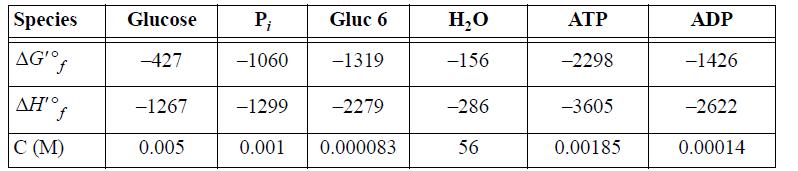
(a) Evaluate the standard state Gibbs energy and enthalpy for Eqn. 18.176 and ΔG′ under the actual concentrations.
(b) Repeat part (a) for Eqn. 18.177.
(c) Biological glycolysis works by coupling the two reactions. Write the overall reaction and evaluate ΔG′ under physiological conditions.
Step by Step Answer:

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira





