The following data have been obtained by graduate student Ron Maurer for the solubility of a crystalline
Question:
The following data have been obtained by graduate student Ron Maurer for the solubility of a crystalline substance (which happens to be a protein, but that is irrelevant) that ionizes on dissolution as a function of the molality of the added salt MgCl2
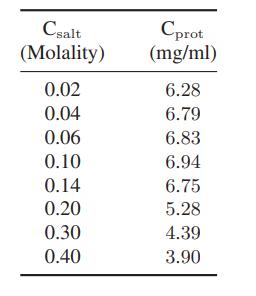 a. Explain qualitatively why the solubility first increases with increasing salt concentration (that is, salts in) and then descreases (i.e., salts out) with still higher salt concentrations.
a. Explain qualitatively why the solubility first increases with increasing salt concentration (that is, salts in) and then descreases (i.e., salts out) with still higher salt concentrations.
b. Write the equations for the thermodynamic based model you would use to correlate the data.
c. What additional information would you need to use the model your suggested in part b. to correlate these data?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





