When propane is heated to high temperatures, it pyrolyzes or decomposes. Assume that the only reactions that
Question:
When propane is heated to high temperatures, it pyrolyzes or decomposes. Assume that the only reactions that occur are
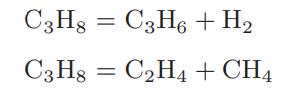
and that these reactions take place in the gas phase.
a. Calculate the composition of the equilibrium mixture of propane and its pyrolysis products at a pressure of 1 bar and over a temperature range of 1000 to 2000 K.
b. Calculate the composition of the equilibrium mixture of propane and its pyrolysis products over a temperature range of 1000 to 2000 K if pure propane at 25°C and 1 bar is loaded into a constant-volume (bomb) reactor and heated.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





