Question: An aqueous mixture that is (30 mathrm{wt} % mathrm{Mn}left(mathrm{NO}_{3} ight)_{2}), initially at (20^{circ} mathrm{C}), is cooled slowly so that it is always at equilibrium. Data
An aqueous mixture that is \(30 \mathrm{wt} \% \mathrm{Mn}\left(\mathrm{NO}_{3}\right)_{2}\), initially at \(20^{\circ} \mathrm{C}\), is cooled slowly so that it is always at equilibrium. Data are in Figure 17-6.
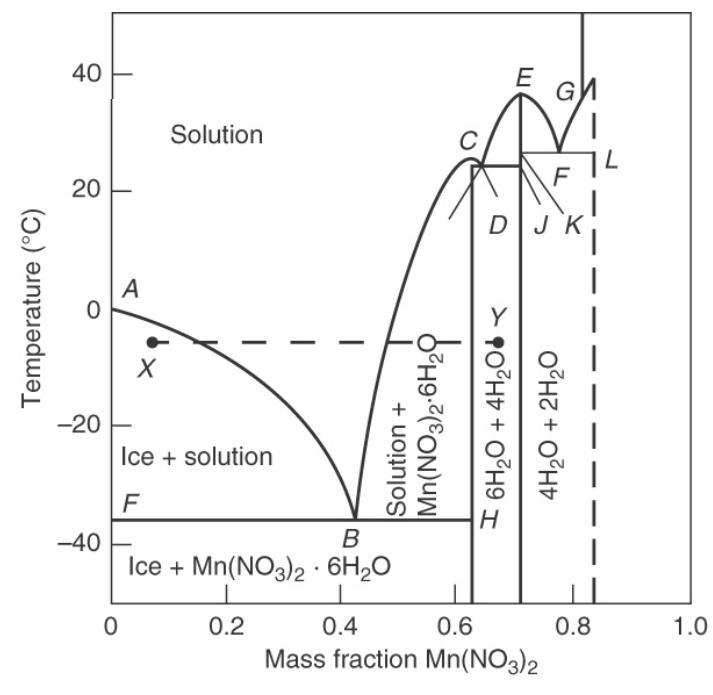
a. At \(0^{\circ} \mathrm{C}\) what phase(s) are present, and what is (are) their composition(s)?
b. At \(-20^{\circ} \mathrm{C}\) what phase(s) are present, and what is (are) their composition(s)?
c. At \(-20^{\circ} \mathrm{C}\) how many \(\mathrm{kg}\) per \(\mathrm{kg}\) of feed do we have of each phase present?
Temperature (C) 40 20 20 Solution E A -20 Ice solution F -40 B Ice Mn(NO3)2 6H2O 0 0.2 0.4 DJ K Mn(NO3)2.6H Solution + 6H2O + 4H2O + 2H2O 0.6 Mass fraction Mn(NO3)2 0.8 1.0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


