Ethylene is dimerized to 1-butene on the surface of a catalyst. At high temperature the reaction kinetics
Question:
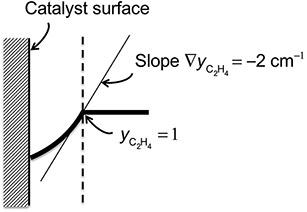
Ethylene is dimerized to 1-butene on the surface of a catalyst. At high temperature the reaction kinetics becomes very fast and the rate of reaction becomes controlled by diffusional mass transfer.
(a) Obtain an expression for the overall reaction rate of ethylene assuming that steady-state diffusion through a thin stagnant gas film adjacent to the catalytic surface controls the process. The reaction occurs only on the catalyst surface.
(b) For the conditions shown in Fig. P.9.3, calculate the rate of reaction of ethylene per unit area of catalyst if the temperature is 100°C, the absolute pressure is 6.6 atm, and the binary diffusivity in ethylene -1-butene mixtures is estimated to be ![]() = 0.0183 cm2/s.
= 0.0183 cm2/s.
FIGURE P.9.3:
Step by Step Answer:

Heat And Mass Transfer For Chemical Engineers Principles And Applications
ISBN: 9781264266678
1st Edition
Authors: Giorgio Carta





