Question: Extraction is almost invariably a ternary mass transfer problem instead of binary because of partial miscibility of diluent and solvent. Typically, as solute is removed
Extraction is almost invariably a ternary mass transfer problem instead of binary because of partial miscibility of diluent and solvent. Typically, as solute is removed from diluent, solvent is less soluble in diluent and must also be transferred to the extract phase. As the extract phase gains more solute, diluent becomes more soluble in the extract, and there will be transfer of diluent into the extract phase. For systems with low partial miscibility (e.g., water-chloroform-acetone in Table 13-6) the amount of additional mass transfer is small, and binary analysis is probably accurate. For systems with significantly more miscibility (e.g., methylcyclohexane-toluene-ammonia at the higher temperatures in Figure 13-15) significant amounts of diluent and solvent can be transferred. Set up, but do not solve the Maxwell-Stefan difference equations for a ternary extraction. Because formation of two liquid layers occurs in nonideal systems, the model needs to use the nonideal form of the Maxwell-Stefan equations. Show how you would calculate \(\Delta \mathrm{x}_{\mathrm{A}}, \Delta \mathrm{x}_{\mathrm{B}}, \mathrm{x}_{\mathrm{A}}, \mathrm{x}_{\mathrm{B}}, \overline{\mathrm{x}}_{\mathrm{C}}\), and \(\bar{ho}_{\mathrm{m}}\). Unfortunately, activity coefficient and diffusivity or mass transfer data required are often not available.
Table 13-6
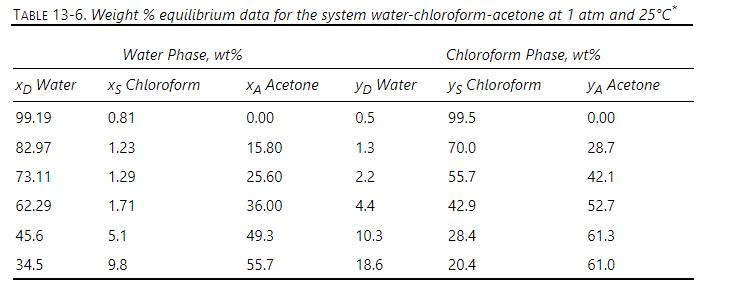
Figure 13-15
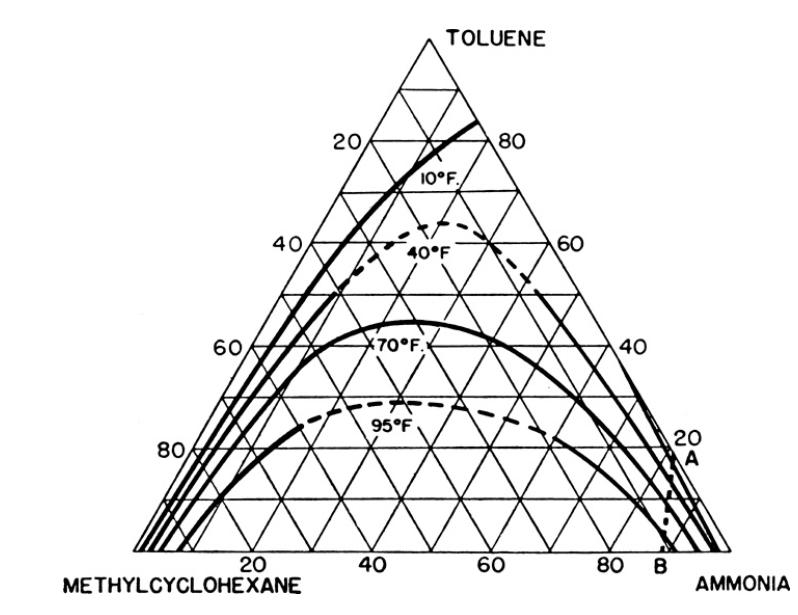
TABLE 13-6. Weight % equilibrium data for the system water-chloroform-acetone at 1 atm and 25C* Water Phase, wt% Chloroform Phase, wt% XD Water Xs Chloroform XA Acetone Water ys Chloroform YA Acetone 99.19 0.81 0.00 0.5 99.5 0.00 82.97 1.23 15.80 1.3 70.0 28.7 73.11 1.29 25.60 2.2 55.7 42.1 62.29 1.71 36.00 4.4 42.9 52.7 45.6 5.1 49.3 10.3 28.4 61.3 34.5 9.8 55.7 18.6 20.4 61.0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


