Question: In Problem (15 . mathrm{H} 5) changing the pressure changes the diffusivities but does not change the Henry's law constant of ammonia. However, changing the
In Problem \(15 . \mathrm{H} 5\) changing the pressure changes the diffusivities but does not change the Henry's law constant of ammonia. However, changing the pressure does change the surface concentrations. Explain.
Problem 15.H5
Repeat Example \(15-8\) but for a pressure of \(1.1 \mathrm{~atm}\). The diffusivities depend on pressure. Also answer Problem 15.A6.
Example 15-8
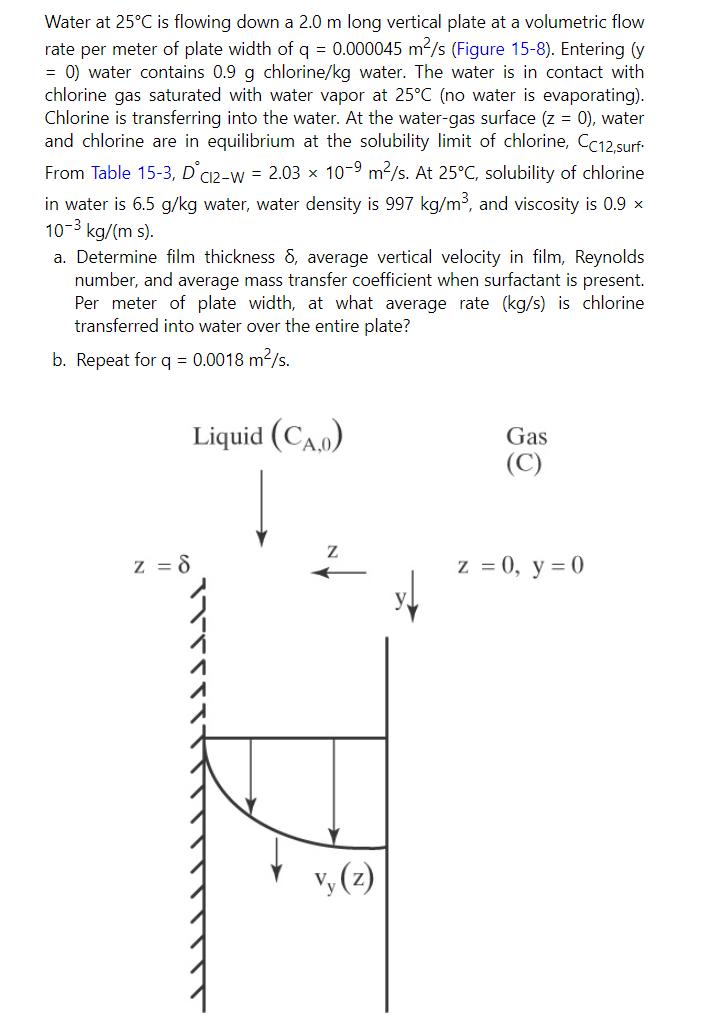
Water at 25C is flowing down a 2.0 m long vertical plate at a volumetric flow rate per meter of plate width of q = 0.000045 m/s (Figure 15-8). Entering (y = 0) water contains 0.9 g chlorine/kg water. The water is in contact with chlorine gas saturated with water vapor at 25C (no water is evaporating). Chlorine is transferring into the water. At the water-gas surface (z = 0), water and chlorine are in equilibrium at the solubility limit of chlorine, CC12,surf From Table 15-3, DC12-W = 2.03 10-9 m/s. At 25C, solubility of chlorine in water is 6.5 g/kg water, water density is 997 kg/m, and viscosity is 0.9 x 10-3 kg/(m s). a. Determine film thickness 8, average vertical velocity in film, Reynolds number, and average mass transfer coefficient when surfactant is present. Per meter of plate width, at what average rate (kg/s) is chlorine transferred into water over the entire plate? b. Repeat for q = 0.0018 m/s. Liquid (CA.0) Gas (C) Z z = 8 vy (z) z = 0, y = 0
Step by Step Solution
3.40 Rating (144 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


