The following data were obtained for the absorption of a sparingly soluble gas A at 20C into
Question:
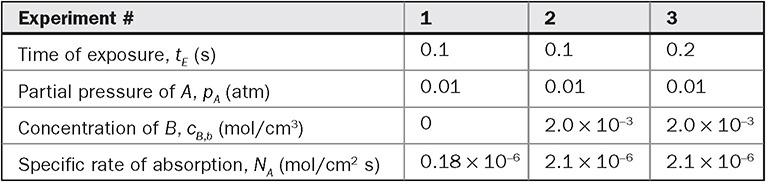
The following data were obtained for the absorption of a sparingly soluble gas A at 20°C into an aqueous solution of a non-volatile reactant B in a laminar jet apparatus as described in Example 16.3. The time of exposure was 0.1 s and the irreversible reaction A + B → P occurred. The reaction kinetics is first order in A and first order in B, the solubility of A is 1.0 × 10−3 mol/cm3 atm, and the diffusivity of B in water at infinite dilution is 2.0 × 10−5 cm2/s. The gas-side resistance is negligible. Based on these data determine:
(a) The diffusivity of A in pure water.
(b) The rate constant for the chemical reaction. Make sure you verify the reaction regime.
(c) The enhancement factor and the specific rate of absorption of A ( ![]() ) at 20°C ifthe partial pressure of A is increased to 1.0 atm, the concentration of B is 4.0 × 10−3 mol/cm3, and the time of exposure is 0.01 s.
) at 20°C ifthe partial pressure of A is increased to 1.0 atm, the concentration of B is 4.0 × 10−3 mol/cm3, and the time of exposure is 0.01 s.
Step by Step Answer:

Heat And Mass Transfer For Chemical Engineers Principles And Applications
ISBN: 9781264266678
1st Edition
Authors: Giorgio Carta





