Question: This problem is long and involves many calculations because it is a hand calculation of the matrix method for nonisothermal absorption. Suggestion: Set up everything
This problem is long and involves many calculations because it is a hand calculation of the matrix method for nonisothermal absorption. Suggestion: Set up everything on a spreadsheet. It makes keeping the calculations procedure accurate and correcting numerical errors easier. An absorber with two equilibrium stages is operating at \(1.0 \mathrm{~atm}\). Feed is \(10.0 \mathrm{kmol} / \mathrm{h}\) of a \(70.0 \mathrm{~mol} \%\) ethane, \(30.0 \mathrm{~mol} \% \mathrm{n}\)-pentane mixture that enters at \(10^{\circ} \mathrm{C}\). Solvent is pure noctane at \(25.0^{\circ} \mathrm{C}\), and solvent flow rate is \(20.0 \mathrm{kmol} / \mathrm{h}\). Find the outlet compositions and temperatures. Column is adiabatic. For the first guess, assume that all stages are at \(21.0^{\circ} \mathrm{C}\), use a DePriester chart for \(\mathrm{K}\) values, and assume flow rates are
\[ \begin{aligned} & \mathrm{L}_{0}=20.0 \mathrm{~V}_{1}=6.00 \mathrm{kmol} / \mathrm{h} \\ & \mathrm{L}_{1}=21.0 \mathrm{~V}_{2}=7.00 \\ & \mathrm{~L}_{2}=24.0 \mathrm{~V}_{3}=10.0 \end{aligned} \]
Then go through one iteration of the sum rates convergence procedure (Figure 12-13) using direct substitution to estimate new flow rates on each stage:
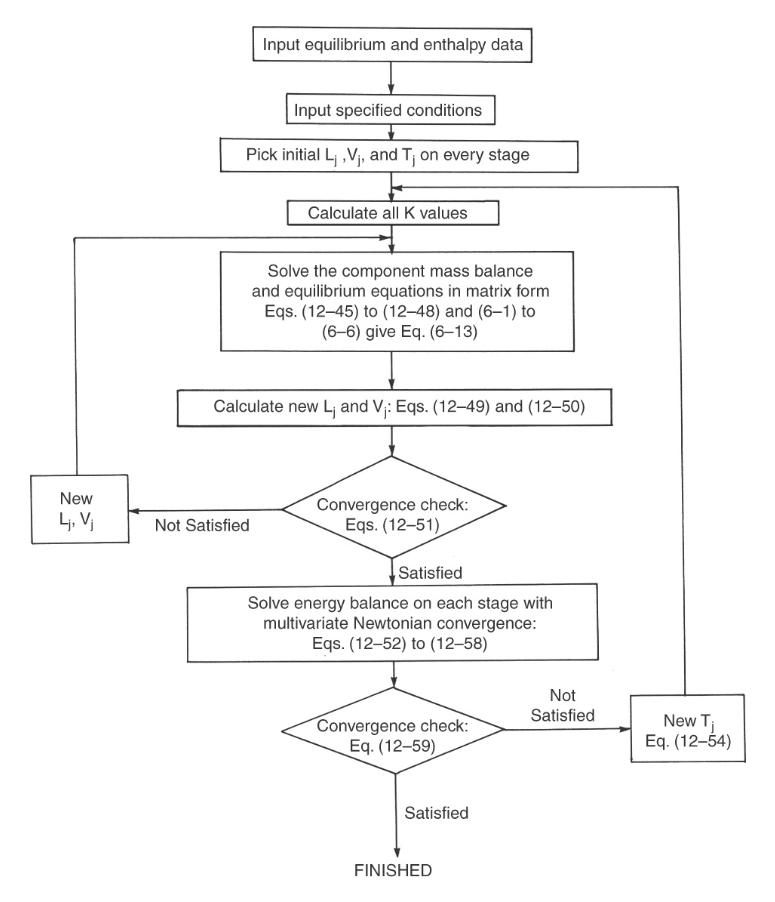
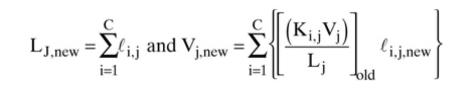
You could use these new flow rates for a second iteration, but instead of doing a second iteration in the flow loop, use a paired simultaneous convergence routine. To do this, use new values for liquid and vapor flow rates to find compositions on each stage.
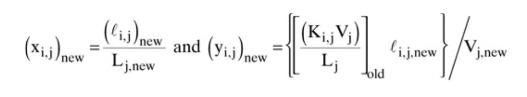
Then calculate enthalpies from pure-component enthalpies given in Table 2-7 in Example 2-3, and use the multivariable Newtonian method [Eqs. (12-52) to (12-58)] to calculate new temperatures on each stage. You will then be ready to recalculate \(\mathrm{K}\) values and solve mass balances for a second iteration. However, for this assignment, stop after new temperatures have been estimated. Assume ideal solution behavior to find the enthalpy of each stream.
Table 2-7
Example 2-3

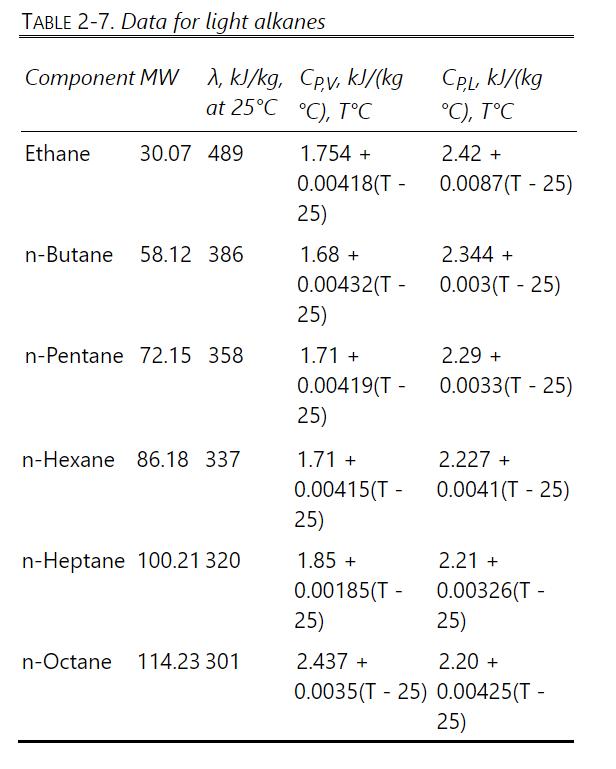
Input equilibrium and enthalpy data Input specified conditions Pick initial L,V, and Tj on every stage Calculate all K values Solve the component mass balance and equilibrium equations in matrix form Eqs. (12-45) to (12-48) and (6-1) to (6-6) give Eq. (6-13) Calculate new Lj and V: Eqs. (12-49) and (12-50) New Lj, Vj Not Satisfied Convergence check: Eqs. (12-51) Satisfied Solve energy balance on each stage with multivariate Newtonian convergence: Eqs. (12-52) to (12-58) Not Satisfied Convergence check: Eq. (12-59) Satisfied FINISHED New Tj Eq. (12-54)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


